 |
| Unsafe drug: Customers are urged not to buy or consume a slimming product named Xin Kang San because it contains undeclared drug ingredients that may trigger heart problems. |
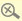
|
The Department of Health has urged customers not to buy or consume a slimming product named Xin Kang San because it contains undeclared drug ingredients.
The department launched an investigation into a complaint received on July 23 and took away the product for analysis. Laboratory results showed the product contains sibutramine and a small amount of fenfluramine.
Sibutramine is a Western drug used as appetite suppressant. However, it can cause increased blood pressure and heart rate, and people with heart problems should not take it. Products containing sibutramine must be registered before they can be sold in Hong Kong.
Fenfluramine was used previously as an appetite suppressant for management of obesity but was banned in Hong Kong in 1998 after studies linked it to heart valve disease.
People who have been using the product should stop using it immediately. They should consult their doctors for medical advice if they feel unwell.
Go To Top
|



