|
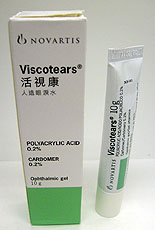
| Drug alert: Artificial tears Viscotears is not registered in Hong Kong and needs to be recalled. |
The Department of Health today instructed Novartis Pharmaceuticals (HK) Ltd to recall artificial tears Viscotears from the market as the product was found to be a pharmaceutical product without registration in Hong Kong.
Manufactured in Germany and registered in some European Union countries, Viscotears is used for dry eyes.
The department inspected offices of Novartis and drug wholesaler Zuellig Pharma Limited which imported Viscotears for Novartis last night.
According to available analysis reports from the importer, there is no immediate safety, efficacy or quality concern over using the product.
It was distributed to 17 public hospitals, some private hospitals, private doctors, pharmacies, drug stores and vet clinics. Doctors and pharmacists should stop dispensing it.
The company has set up a hotline 2881 4259 to answer clients' enquiries from 9am to 5pm daily.
The Department of Health has also set up an enquiry hotline - 2319 2905 - which operates from 9am to 5pm tomorrow onward.
|