 |
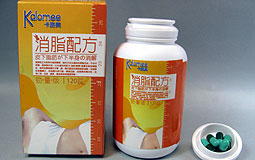
| Total recall: Kalomee contains an undeclared sibutramine analogue that can cause serious side effects. |
|
The Department of Health warns the public not to consume slimming product Kalomee as it contains an undeclared drug analogue which can cause serious side effects.
Laboratory tests found a sibutramine analogue in the product sample obtained from a Tuen Mun beauty parlour.
Sibutramine is used as an appetite suppressant. Side effects include increased blood pressure and heart rate, psychosis and convulsion. People with heart problems should not take it.
Sibutramine analogues are chemically similar to sibutramine and are expected to have the same side effects.
The wholesaler has been instructed to recall the product. Call 3106 8091 for enquiries.
Go To Top
|



