 |
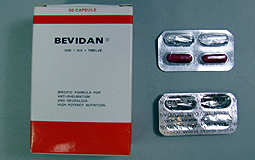
| Drug alert: Bevidan contains prednisolone and mefenamic acid, which must be registered under the Pharmacy & Poisons Ordinance before sale. |
|
The Department of Health urges people not to buy or use health product Bevidan which contains undeclared Western drug ingredients which can cause side effects.
A 59-year-old man suffered from high blood pressure, high blood sugar and mild muscle weakness in his upper limbs after taking the product to ease muscle pain. He is now stable.
The product was found to contain prednisolone, a steroid which can cause high blood pressure, high blood sugar and peptic ulcers, and mefenamic acid, a non-steroidal anti-inflammatory drug which can cause nausea, stomach pain and bleeding.
Products containing Western drug ingredients must be registered under the Pharmacy & Poisons Ordinance before sale. Possession or sale of unregistered pharmaceutical products warrants a $100,000 fine and two years' jail.
Go To Top
|



